Tissue Engineering for Anterior Cruciate Ligament Reconstruction a Review of Current Strategies
Impact Statement
The poor healing of tendon and bone is an important problem that puzzles the postoperative recovery of ligament reconstruction. Usually, subsequently arthroscopic surgery, there is a gap betwixt the graft and bone healing in the bone tunnel. Therefore, researchers promise to obtain improve healing effect through other ways such equally tissue applied science, and then every bit to improve the role recovery of ligament and joint postoperatively. In this newspaper, a systematic summary of the in vivo research was carried out, hoping to summarize the research achievements and find new inspiration and quantum to inspire futurity research.
Introduction
Tendon defect is one of the common clinical diseases (Hayashida et al., 2018; Kunze et al., 2019). After the tendon injury, it often leads to limb dysfunction, severe cases, or even inability without repairation in time (Ando et al., 2019). Tendon-bone healing is an important cistron in determining the success of ligament reconstruction (Li et al., 2019; Huang et al., 2020). Whether it is articulatio genus cruciate ligament, lateral collateral ligament, posterolateral complex reconstruction, shoulder joint sleeve repair, or ankle ligament reconstruction, the degree of tendon-bone healing directly affects the postoperative rehabilitation process and surgical effect (Zhao et al., 2019). Studies take shown that in addition to surgical errors, failure to attain strong tendon-os healing is the master reason for failure of ligament reconstruction surgery (Han L. et al., 2019; Zhang et al., 2020). Tendon injuries can be divided into two categories: non-defective injuries and defective injuries (Van Der Made et al., 2019; Wisbech Vange et al., 2020). For the tendons with defects and injuries, several handling methods are available, such equally autogenous tendon transplantation, allogeneic tendon transplantation, heterogeneous tendon transplantation, and bogus tendon replacement (Periasamy et al., 2019). In the former, due to lack of donor tendon or immune rejection, the tendon transplantation is restricted. With the development of prison cell culture technology and transplantation technology and the development of biomaterials science, a new ideal tendon replacement-tissue engineered bogus tendon with synthetic materials, will somewhen solve the problem of repairing defective tendons (Khoo and Nikkhah, 2019).
Tissue engineering scaffold mainly serves the following aspects: (1) As a framework connecting cells and tissues, information technology tin can be used to guide tissues to abound into a specific shape (Lewandowska-Lancucka et al., 2015). (two) Every bit a carrier of signaling molecules, it is transported to the defect site, and as a dull-release torso, the osteoinductive factor slowly acts. (3) As a identify for os tissue to reproduce, differentiate and metabolize, ship nutrients for jail cell growth, and eliminate waste. (4) The specific sites on the surface of the scaffold react specifically with the cells and play the role of "identification" and selective adhesion to unlike types of cells. Information technology can be seen that the scaffold plays an extremely important role in os tissue engineering, which not but plays a physical role in connecting and supporting cells and tissues simply also regulates diverse functional activities of cells. Tendon/bone tissue engineering science has high requirements for scaffold materials, and an ideal in vivo graft should run into the following points. For case, the scaffold materials must be non-toxic and take skillful biocompatibility (Karel et al., 2018). The textile must also be biodegradable and can be gradually degraded and metabolized in the body as the cells proliferate, and then be absorbed (Hejbol et al., 2017). Also, the degradation products of the materials must exist not-toxic, with good biocompatibility, and will not adversely impact tissues and organisms. In addition, the material must have good processing properties and tin can be processed into the required shape and structure like open-pore structure and proper pore size. The scaffold must have expert cell affinity, suitability for prison cell adhesion, proliferation, and secretion of the matrix, as well as sure mechanical backdrop, including strength and flexibility. Under conventional sterilization weather condition, the scaffold must be able to withstand sterilization without physical, chemic, and biological changes. Moreover, the scaffold should not only maintain its shape during the cell culture operation but besides be able to withstand the surgical operations implanted in the trunk, to ensure that it will not break during the functioning, tin can fit with the body, and will not cause mechanical damage to the torso tissue.
In contempo years, studies on improving tendon-os healing have mainly focused on promoting ligament-bone integration at tendon sites (Xu et al., 2019). This includes the apply of periosteum, biogels, scaffolds, growth factors, stem cells, or other reconstruction materials that promote bone growth or ligament attachment points (Uz et al., 2019; Huang et al., 2020; Rodriguez-Vazquez and Ramos-Zuniga, 2020; Sadeghinia et al., 2020). However, reviews describing the awarding of tissue applied science scaffold, particularly biomaterials, in tendon-os healing in vivo are defective. The purpose of this systematic review was to (1) critique the bear witness in vivo regarding the use of tissue engineering scaffold to accomplish tendon-bone healing; (2) provide a descriptive summary of the current evidence for tissue engineering scaffold use in tendon-os healing peculiarly biomaterials, as this is the first systematic review on the topic; and (iii) highlight areas of futurity research to facilitate clinical application.
Methods
Eligibility Criteria
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Maher et al., 2003). This review included original peer-reviewed studies based on the following inclusion criteria: (1) publication in an English-linguistic communication journal; (2) an in vivo creature study or clinical study that mainly evaluated the use of issue engineering scaffold in tendon bone healing; and (three) scaffold which was manufactured using biomaterials. Studies reporting in vitro piece of work without in vivo analysis were excluded. Only original peer-reviewed articles were included, and so letters, editorials, review articles, conference, patents, and coming together abstracts and studies not involving tissue engineering scaffold were excluded.
Literature Search Strategy
Literature searches were conducted in 3 electronic databases (PubMed, EMBASE, and Web of Scientific discipline) from 1 Jan 1990 to 31 Dec 2019. The following search terms were used for the literature search: "(tissue engineering OR tissue engineering scaffold OR scaffold for tissue engineering OR scaffold) AND (tendon-bone healing OR tendon-os OR healing of bone-tendon) AND (vivo OR homo OR patient OR animal OR mouse OR rat OR rabbit OR dog OR sheep OR sus scrofa OR horse OR ovine)." Because the telescopic of this review was big in terms of event measures, a systematic review, not a meta-analysis, was performed.
Study Option
The manufactures were initially screened to appraise suitability for inclusion according to the criteria with their title and abstract, and then the total text of each article was downloaded to define the relevance of the piece of work for investigators. During selection, the following information was extracted: details of the animal model or clinical data, the groups investigated, the types of scaffold, the methods of evaluation, and the principal findings. The selected manufactures in the written report were reviewed, evaluated, and discussed by the authors. A senior investigator would make the final determination if the reviewers were non able to reach a consensus agreement on the inclusion of any articles.
Results
Search Results
Figure i represents the process for evaluating studies for inclusion in the systematic review. A full of 506 articles were identified through our search. Afterwards removal of duplicates, every bit well every bit messages, editorials, review articles, briefing, patents, meeting abstracts, and involved studies, 189 manufactures were screened for eligibility by means of title and abstract. Following the exclusion of 112 articles, 77 full-text manufactures were assessed for eligibility. Finally, 27 studies were identified and included in our analysis, which were consisted of ii homo trials and 25 animal studies. Furthermore, the 27 studies using this retrieval strategy were analyzed.
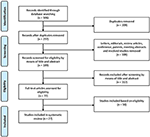
Figure ane. A flowchart showing the selection of studies for inclusion in the systematic review.
Data Extraction
Information of data extraction of the 27 studies is available in Table i, covering details of the animal model or clinical information, the groups investigated, and the types of scaffold. Of the 27 included in vivo studies, two were human trials (Petruskevicius et al., 2002; Mochizuki and Ochi, 2015) and 25 were animal studies. More than one-half of the beast studies (n = 14) utilized a rabbit model (Zhang et al., 2011; Li et al., 2012, 2017; Jiang et al., 2014; Han et al., 2015; Chou et al., 2016; Chung et al., 2017; Lee et al., 2017; Cai et al., 2018; Hu et al., 2018; Chen P. et al., 2019; Han F. et al., 2019; Learn et al., 2019), eight studies used rats (Loeffler et al., 2013; Zhao et al., 2014, 2015a,b; Kovacevic et al., 2015; Zhang et al., 2018; Zhu M. et al., 2019; Zhu Q. et al., 2019), and iii studies used pigs (Fleming et al., 2009; Vavken et al., 2012; Li et al., 2014). The involved patients and animals were mature, and no modest or immature fauna model had been investigated in previous studies so far as searched. Amongst the two human trials, only ane had tibia defect, which was repaired with Osteoset (Petruskevicius et al., 2002), the other had not indicate out the defective function (Mochizuki and Ochi, 2015). In fauna models, the defect occurred in supraspinatus tendon, infraspinatus tendon, inductive cruciate ligament, long digital extensor tendon, achilles tendon, and proximal tibial metaphysis. Moreover, there were xv studies that only used synthetic scaffolds like poly lactide-co-glycolide (PLGA), collage, polycaprolactone (PCL), polylactic acid (PLA), and polyethylene terephthalate (PET) to repair the defect of tendon-bone interface, and the rest of the 12 studies used biological interventions, which combined cells or jail cell factors to raise the tendon os healing, such as bone morphogenetic protein (BMP), stromal cell-derived gene (SDF), ligament-derived stalk/progenitor cells (LSPCs), kartogenin (KGN), platelet-derived growth factor (PDGF), mesenchymal stalk jail cell (MSC), platelet, etc.
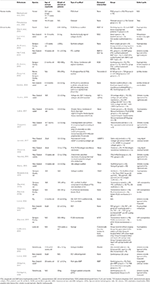
Tabular array i. The information of data extraction of the included studies.
Safety and Efficacy Evaluation
The adverse events, histological and biomechanical results, and data from other tests were assessed to determine the scaffold prophylactic and efficacy (Table ii). Equally for the adverse events, no complications were encountered; all the participants and animals recovered well, and macroscopic inspection of the collected specimens did not reveal any gross infectious or inflammatory changes in most of the included studies in vivo. Only one rabbit with cocky-inflicted wound developed dehiscence and heterotopic of bone formation in the report of Learn et al. (2019), and one animal with minor harm upon tricalcium phosphate (TCP) insertion was institute in the report of Li et al. (2014). As for the histological results, information technology was demonstrated that the tendon-bone integration at the interface using synthetic scaffolds was better than transosseous or direct repair and could be enhanced by biological interventions. The healing was generally characterized by collagen system comeback, glycosaminoglycan deposition, new bone germination (mineralization), fibroblast-like cells, chondrocyte-similar cells, and immature neo-enthesis structure increasement. As for biomechanical tests, the results of ultimate strength to failure, stiffness, and force were extensively investigated and compared, which suggested that the tendon-bone healing with scaffolds produced superior biomechanical outcomes to transosseous or straight repair, especially combined with biological interventions. In addition, other tests were performed to evaluate the efficacy of tissue engineering scaffold in tendon bone healing, such every bit micro-CT, JOA score, and mRNA level, whose results were consistent with the histological results.
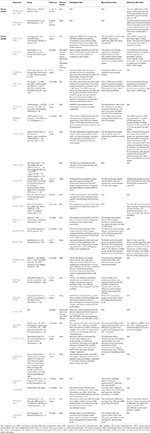
Table 2. The safety and efficacy of tissue engineering scaffold in tendon bone healing in vivo.
Discussion
With the evolution of biomaterials science, the tissue engineering scaffold plays an extremely important office in tendon-bone healing. This systematic review has proved the efficacy and prophylactic of constructed scaffolds in vivo, which can be enhanced by biological interventions. Notwithstanding, the previous researches are mainly focused on animate being models, not homo trials which limits the clinical application of these scaffolds. In this review, we provide a descriptive summary of 27 articles related to the use of tissue engineering scaffold specially biomaterials in tendon-bone healing to better guide the clinical practice.
Tendon-Bone Healing Procedure
Normal tendon-bone junctions are divided into indirect insertion and straight insertion (Mochizuki et al., 2006, 2014; Iwahashi et al., 2010; Sasaki et al., 2012). Indirect insertion refers to the thick fibrous tissue that directly connects the tendon or ligament to the periosteum, such as the medial collateral ligament tibial point (Mochizuki et al., 2006, 2014; Sasaki et al., 2012). Straight insertion refers to "anchoring" soft tissue to the deep layer of bone through typical fibrous cartilage tissue (Iwahashi et al., 2010). Its straight embedding point is a four-layer tissue transformation surface area, including bone, calcified cartilage, non-calcified cartilage, and ligament tissues, such as anterior cruciate ligament and rotator cuff. At present, the related research on the surgical technique and fixation method of tendon or ligament reconstruction aims to reach the maximum contact expanse betwixt the tendon and bone during reconstruction, ensure the stability and tightness of the contact surface, and minimize the influence of external forces. When the tendon is in contact with the bone, through direct or indirect tendon-bone healing, a normal tendon-bone interface is formed, a stable connexion is established, and the reconstructed ligament functions. Direct tendon-bone healing refers to a tendon-bone connection with four-layer structure, which exists betwixt the tendon and the tunnel opening after healing (Iwahashi et al., 2010; Sasaki et al., 2012). Indirect tendon-bone healing refers to the connection between tendons and bones through Sharpey-similar fibers (Mochizuki et al., 2006, 2014). This blazon of healing takes a long time, and the tensile force between tendons and bones subsequently healing is lower than that of direct healing.
An animal experiment showed that (Weiler et al., 2002), after the tendon is implanted in the bone tunnel, it needs to get through the necrosis period, the proliferation period, and the ligamentization period to attain true healing. The period of necrosis is also called early healing, and more often than not refers to the first 4 weeks after the implant is implanted. During this process, the graft has necrosis, and its mechanical strength is significantly lower than that of the implant. The proliferative stage refers to the remodeling, revascularization, and strong cell activeness of the graft from 4 to 12 weeks after surgery. The ligamentization menstruation refers to the menstruum from the proliferation period to the end of graft remodeling, which can be as long as one-half a year to several years. Tabuchi et al. (2012) observed the tendon implanted in the rabbit bone tunnel for 26 weeks; the results showed that Sharpey-like fibers containing blazon III collagen fibers were gradually replaced by type I collagen from 12 weeks later on surgery until the fibers in the graft formed a connection with Sharpey-similar fibers at week 26.
Biomaterials of Tendon-Bone Healing and Bone Tissue Technology Scaffolds
Biomaterials of tissue technology scaffold is an of import and difficult point in the enquiry of tissue engineering. Without suitable scaffolds, the seed cells will be lost and die. Tissue engineering scaffold materials should have skillful biocompatibility, biodegradability, iii-dimensional construction, plasticity, and equivalent mechanical strength. It also has good surface action, which is conducive to the adhesion of seed cells and provides a proficient microenvironment for the growth and reproduction of cells on the surface and secretion matrix. For tendon and tissue engineering science scaffold materials, the most researched at present are natural materials, synthetic materials, copolymers, and composite materials. The following are commonly used and investigated in this review.
Natural Materials
The fantabulous biocompatibility of natural polymers such as collagen and fibrin can significantly improve the interaction betwixt materials and tissue cells (Doty et al., 2019). In our review, collagen is widely used and evaluated in tendon bone healing. It is the chief component of extracellular matrix (ECM), which can exist extracted from animate being bones and fascia through digestion, hydrolysis, and other processes (Shi et al., 2019; Mohammed et al., 2020). In the process of evolution, collagen retains the original amino acid sequence, and the scaffold material made past information technology has not-antigenicity, adept biocompatibility in vivo, and permeability (Dunshee et al., 2020). Because the tissue composition of tendons is mainly composed of collagen fibers, the big fiber bundles are arranged in parallel, and their direction is consistent with the traction forcefulness they bear. Collagen cobweb has toughness and strong traction resistance; in add-on, the cell adhesion signal sequence contained in it tin can besides guide the specific identification of the scaffold material by the cells. Collagen fiber has toughness and stiff traction resistance (Sharma et al., 2019; Tonndorf et al., 2020). Subsequently years of development, its preparation method has been quite mature. It has at present been certified by the US Nutrient and Drug Administration (FDA) and tin can be successfully used in ECM scaffolds for tissue-engineered tendons. Bellincampi et al. (1998) inoculated autogenous tendon cells into collagen scaffolds and implanted them in rabbit knee joint joints and subcutaneously. The circuitous was withal visible after 8 weeks. Young et al. (1998) used autologous os MSC to adsorb on collagen gel and planted the chemical compound on the joint of tendon. It was plant that the tendon treated with MSC was thicker than the control group, and the collagen fiber assembly, seam characteristics, and load operation were better than the command group. In the included studies, the collage has been used as tissue engineering scaffold for tendon bone healing in many animal models (Fleming et al., 2009; Vavken et al., 2012; Bi et al., 2015; Kovacevic et al., 2015; Lee et al., 2017; Hu et al., 2018; Learn et al., 2019; Zhu One thousand. et al., 2019), and its efficacy can exist improved by utilizing a biofabrication process known equally electrocompaction. Learn et al. (2019) had used electrochemically aligned collagen (ELAC) threads woven into biotextile scaffolds as grafts to repair disquisitional infraspinatus tendon defects in New Zealand white rabbits, and it was found that woven ELAC scaffolds was intact and mechanically competent post-obit 3 months of implantation and biological characteristics of tendon were present in the tissue around and inside the scaffolds.
Acellular scaffolds play a unique role in promoting tendon bone healing. Lu et al., 2019 plant an advisable decellularization protocols for fibrocartilage tissue and prepared a novel book-shaped acellular fibrocartilage scaffold with jail cell-loading adequacy and chondrogenic inducibility for tissue-engineered fibrocartilage and bone-tendon healing. This screened scaffold lonely could induce endogenous cells to satisfactorily regenerate fibrocartilage at 16 weeks, as characterized by fibrocartilaginous ECM deposition and good interface integration and may have further broad clinical applications in promoting bone-tendon healing. And then they creatively prepared ii kinds of acellular scaffolds from bone or fibrocartilage tissue, namely book-type acellular bone scaffold (BABS) and book-type acellular fibrocartilage scaffold (BAFS). Histologically, both scaffolds well preserved the natural ECM construction without cellular components. In vitro studies have shown that BABS has a good ability of osteogenic induction, while BAFS has a good power of cartilage induction. Using a rabbit partial patellectomy model, both BAFS and BABS can promote tendon-os healing, while the BAFS was more conductive in tendon-bone healing than the BABS. This study may provide a valuable reference for screening the optimal tissue-engineering scaffold practical in tendon-bone healing (Lu et al., 2019).
Synthetic Materials
Synthetic materials similar PLA, polyglycolic acid (PGA) possess iii master structural forms (fiber scaffold, porous foam, tubular structure). The deposition products of PLA and PGA are lactic acid and glycolic acrid, respectively, which are intermediate metabolic products of the tricarboxylic acid wheel. They accept good biodegradability and compatibility and volition not crusade inflammation, immune reactions, and cytotoxic reactions. The most widely used biodegradable biomaterials have been widely used in tissue engineering such as bone, cartilage, claret vessels, nerves, and skin (Larn et al., 2019). Cao et al. (2002) inoculated tendon cells obtained from the tendon tissues of dogie shoulders and knees on a cable-like PGA mesh scaffold and implanted them subcutaneously in nude mice after 1 week in vitro culture. It was found that at 12 weeks, tendon tissue similar to the normal tendon structure was formed, and information technology had a certain degree of biomechanical backdrop. Cao et al. (2002) also used the method of autologous tendon cells + PGA + biofilm wrapping to repair the iv cm tendon defect in Leghorn muscles. The results showed that the implanted tissue-engineered tendon was modest, similar to the normal tendon just in full general morphology and histology, and its biomechanical backdrop also reached 83% of the normal tendon.
Moreover, PCL, as a synthetic material, can also be used as scaffold to repair the defect of tendon bone, and many studies also have proved the awarding of PCL (Han et al., 2015; Zhao et al., 2015b; Han F. et al., 2019; Li et al., 2019). PCL is a kind of thermoplastic crystalline polyester obtained by band-opening polymerization of caprolactone with diol as initiator, containing many methyl groups, so information technology has hydrophobicity. In addition, its degradation rate is lower than other aliphatic polyesters (Hagg et al., 1991; Crair et al., 1998). Han F. et al. (2019) have found that PCL scaffold can be loaded with biological interventions such equally BMP-ii and SDF-1α to form fibrous scar tissue and new os for tendon os healing. On other manus, the use of PET is also investigated in previous studies (Li et al., 2012; Jiang et al., 2014). PET is a crystalline-saturated polyester with first-class physical and mechanical backdrop in a wide temperature range. Before, PET was mainly used in the preparation of LARS artificial ligament. Because of its poor hydrophilicity and lack of bone conductivity, it is not conducive to the growth of autogenous bone tissue, thus affecting the tendon-bone healing of the tendon in the bone tunnel and inducing ligament loosening which causes operation failure in the middle and long term after operation (Thian et al., 2006). At the aforementioned time, fibroblasts and synoviocytes cannot abound on the surface of ligament articulation cavity and wrap LARS ligament to induce autologous tissue growth as the poor cytocompatibility of PET fabric, which will eventually lead to article of clothing and fatigue fracture, and the shedding of wear particles will crusade serious synovitis of knee joint. To solve this problem, Jiang et al. (2014) combined silk fibroin (SF) and hydroxyapatite (HAp/HA) blanket by biomimetic route on the surface of PET artificial ligament; the results showed that this combination could induce graft osseointegration in the bone tunnel. Moreover, Li et al. (2012) had proven that surface coating with an organic layer-by-layer self-assembled template of chitosan and hyaluronic acid on a PET artificial ligament could be designed to promote and raise the graft-to-os healing later on artificial ligament implantation in a os tunnel.
Copolymer
The PLA and PGA copolymers include PLGA, poly(D-lactic acid) (PDLA), poly (Fifty-lactic acid) (PLLA), poly(D,L-lactic acid) (PDLLA) (Kikuchi et al., 2018). Since PLGA has first-class biocompatibility and tunable mechanical and degradation properties, it is the most frequently used applications of PLA and PGA in tendon tissue engineering. PLGA not only has skillful biocompatibility simply can also induce the upregulation of certain genes (Fujimaki et al., 2020). The deposition rate tin can also be controlled by changing the ratio of PLA to PGA and combining the loftier deposition rate of PGA and the high strength of PLA. Therefore, PLGA can also be used as a jail cell scaffold for bogus tendons. Fujimaki et al. (2020) and Lim et al. (2019) used PLA and PGA compound PLGA as a stent and implanted a 10-mm defect into the autogenous tendon. The control group was only replanted with PLGA. Test afterward 4 weeks revealed that the cell content was significantly reduced compared with that at two weeks, and collagen fibers I and Three were formed, and the experimental group was more than obvious than the command group. The material was basically degraded at 8 weeks, and the defect was repaired well at 12 weeks, with no lymphocyte infiltration. The biomechanical strength of the experimental group was significantly higher than that of the control group, close to normal tendons. Cai et al. (2018) prepared a dual-layer aligned-random nanofibrous scaffolds SF/P(LLA-CL) using SF-blended poly(L-lactic acid-co-east-caprolactone) (PLLA-CL), which could effectively augment the tendon-to-bone integration and improve gradient microstructure in a rabbit extra-articular model by inducing the new bone formation, increasing the area of fibrocartilage, and improving collagen organization and maturation.
Composite Materials
Natural materials such equally collagen accept good biocompatibility, but at that place are defects such as poor mechanical backdrop, very fast degradation, and poor processing performance. Synthetic materials such as high-molecular materials have defects such as low degradation rate, inflammation caused by acidic degradation products, and minor mechanical properties. Thus, it is difficult for a single type of material to meet the requirements of extracellular matrix materials for tendon-bone healing. These problems can be solved by the principles and methods of blended materials. That is to say, two or more than kinds of biomaterials with complementary characteristics are combined in a sure proportion and manner in society to construct a new blended fabric that can run across the requirements. Researchers combined several single materials through appropriate methods, comprehensively considered the advantages and disadvantages of organic materials and inorganic materials, and complemented each other to form organic/inorganic composite materials, which achieved skilful results in practical applications. Devin et al. (1996) equanimous HAp and PLA/PGA copolymer (fifty:50) into a porous blended matrix cloth and establish that the compressive elastic modulus of the composite increases with the HAp limerick. Therefore, the introduction of calcium-phosphorus ceramics into the PLA/PGA copolymer can improve the shortcomings of poor mechanical backdrop, fast deposition rate, and weak bone binding ability of the PLA/PGA copolymer. Besides, enquiry by Serre et al. (1998) confirmed that porous materials equanimous of type I collagen, chondroitin sulfate, and HAp/HA tin can promote the attachment and growth of osteoblasts and promote the calcification of their secreted matrix. Li et al. (2017) developed a dual-layer organic/inorganic flexible bipolar fibrous membrane (nHA-PLLA) to repair the defect of upraspinatus tendon in New Zealand rabbit, which could act as a bridge between the repaired tendon and footprint, affecting the healing process in multiple means. In other studies, Chung et al. (2017) successfully fabricated a biodegradable and constructed tri-component graft consisting of porous poly(1,8-octanediol-co-citric acrid)-hydroxyapatite nanocomposites (POC-HA) and poly(l-lactide) (PLL) braids, Han et al. (2015) developed a biomimetic nanofiber membrane of polycaprolactone/nanohydroxyapatite/collagen (PCL/nHAp/Col), and Li et al. (2014) prepared a silk-TCP-PEEK scaffold of silk, TCP, and polyether ether ketone (PEEK) to improve the healing and regeneration process of the tendon-bone defect. Zhang et al. (2018) found that trichostatin A (TSA) incorporated aligned fibers of PLLA had an additive effect in directing tenogenic differentiation and confirmed that composite scaffold promoted the structural and mechanical properties of the regenerated Achilles tendon, which provides fresh insights into the regulation of tendon differentiation and a clinically applicable therapeutic approach for tendon regeneration.
Bionic Scaffold
Bionic scaffolds take been of knee interest in tissue engineering which are sufficient for tissue regeneration. Liu et al. (2017) adult a bionic random-aligned-random-tendon ECM composite scaffold for reconstruction of the soft tissue-bone junction in rabbit model. Microcomputed tomography (micro-CT) and histological analysis showed that the bionic scaffold enhanced tendon bone healing and fibrocartilage germination. These results demonstrated that the bionic scaffold could be a promising scaffold for ligament/tendon-os junction repair. Lipner et al. (2015) used bionic scaffolds implanted with pluripotent cells to promote tendon-to-bone healing by promoting collagen degradation and bone formation. Adipose-derived stromal cells were implanted into the repair site of rat rotator gage model to construct nanofiber polylactic acid-glycolic acid copolymer scaffolds with different mineral content gradients. Histologically, the healing interface of all groups was mainly fibrovascular scar reaction. The results showed that the tendon-to-bone healing was dominated by scar germination, which prevented any positive effect of the implanted biomimetic scaffold. In a word, the role of biomimetic scaffold in promoting tendon bone healing is not very clear, and more than studies are needed to prove its positive consequence.
Preparation Method of Scaffolds
Porous and 3-dimensional (3D) scaffolds take been extensively used as biomaterials in the field of tissue engineering in vitro study of jail cell-scaffold interactions. There are many methods for preparing porous materials, such as particle filtration method, melt molding method, emulsion freeze-drying method, high-pressure gas expansion method, cobweb three-dimensional interweaving method, phase separation method, etc. (Freedman and Mooney, 2019). Some scholars try to use two or more methods together, or meliorate existing methods, optimize process parameters, in club to obtain a improve pore structure. Harris et al. (1998) used the gas-foaming method combined with the particle filtration method to gear up the stent fabric with open large pores, which effectively overcome the disadvantages of closed pores. Mikos et al. (1993) applied lamination technology to prepare PLA and PGA into a three-dimensional polymer foam with a certain shape. The micropores of the laminated layers communicate with each other to form a continuous cell construction. These are conducive to cell growth, survival, and proliferation. An intelligent processing engineering science called rapid prototyping has accomplished rapid development in this field (Szlazak et al., 2019). The technology is based on the principles of dispersion and stacking. The three-dimensional model is constructed by performing layer processing on the images obtained by CT or magnetic resonance scanning of the homo body, and then layered and sliced. Finally, the STL format file is transferred to the rapid prototyping auto for processing, and the diverse cross-sectional contours are formed by means of hot melting and cutting, and gradually stacked into a three-dimensional office. This method can exist tailored co-ordinate to the requirements of different patients and has the characteristics of being fast and flexible, so it has great advantages in the processing of tissue engineering scaffolds (Duty et al., 2007; Ravichandran et al., 2017).
Biodegradation
In the past 10 years, the rapid deposition of various materials in the man torso is still a major trouble hindering its clinical application. Considering the interface of tendon bone connection involves 2 different biological structures, the platonic biomaterial used for tendon bone healing should have a college standard. In other words, information technology is necessary to ensure that the stress at the bone-tissue interface can be reduced while matching with the deposition charge per unit during bone healing menstruation, and the enzymatic hydrolysis in vivo at the finish of the tendon can exist resisted while the degradation products will not cause changes of the internal environment pH. Therefore, the widely adult biphasic and triphasic stents are very competitive (Atesok et al., 2016; Tang et al., 2020; Zhou et al., 2021). The degradation rate could be adjusted by ultrasonic pretreatment, and the electrostatic allure or physical cross-linking between the molecules could be used to improve the stability of the materials and add cross-linking agents to make the internal molecular chains of the composites produce stronger cantankerous-linking.
Determination
Functional fibrocartilage regeneration is a choke point in tendon os healing, and the currently bachelor tissue-engineering strategies for fibrocartilage regeneration are bereft because of a lack of advisable scaffold that tin load large seeding cells and induce chondrogenesis of stem cells. Numerous strategies have been employed to improve tendon bone junction healing, including commitment of stem cells, bioactive factors, and synthetic materials, just these are frequently inadequate at recapitulating the complex structure-function relationships at native tissue interfaces Based on the results of several studies, it is found that the challenges that may be faced in the clinical process of tendon bone healing include the post-obit: the regeneration strategy may exist overwhelmed past natural scar-mediated responses, BMP2 is not an effective growth factor to promote tendon bone healing, and scaffold materials may have a negative effect on tendon os healing.
At present, except for the periosteum which has been used clinically and the curative effect is clear, the remaining methods to promote tendon-bone healing have no definite conclusions, and farther research is needed to provide a more stable healing issue after ligament or tendon repair and reconstruction. Although a number of tissue engineering scaffolds accept been developed and investigated, whose efficacy and safety has been proved and can be enhanced past biological interventions in this review, the researches are mainly focused on animal models which are with limitations in clinical application. On the other mitt, tendon tissue engineering science has high requirements for scaffold materials, and the development and research of composite materials will go on to be a hotspot for future research. Therefore, substantial clinical trials remain to be done, and continued progress in overcoming electric current tissue engineering science challenges should permit for successful clinical exercise, which is as well one of the main directions of tissue engineering material enquiry and development in the time to come.
Information Availability Statement
The original contributions presented in the study are included in the commodity/supplementary material, further inquiries can exist directed to the corresponding authors.
Writer Contributions
ZM, BF, and XW: substantial contributions to the formulation or design of the work, or the acquisition, analysis, or estimation of information for the work. XH, JG, ZS, BX, MY, and ZC: drafting the piece of work or revising information technology critically for important intellectual content. DJ and JY: terminal approval of the version to be published, agreed to be accountable for all aspects of the piece of work in ensuring that questions related to the accuracy or integrity of whatsoever part of the piece of work are appropriately investigated and resolved.
Funding
This piece of work was supported by Peking University Health Science Center (Nos. A63498-24 and BMU2018 MX023), by Peking Academy Third Hospital (Nos. BYSY2018004 and BMU2019GJJXK012), and by the National Fundamental R&D Plan of Red china (No. 2017YFB1303000).
Conflict of Involvement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would similar to give thanks Dr. Xing Wang of the Institute of Chemistry, Chinese Academy of Sciences for his suggestions and technical support.
References
Ando, J., Sakuraba, Thousand., Sugawara, A., Goto, A., Azuma, South., Mitsuhashi, N., et al. (2019). Free flap reconstruction of Achilles tendon and overlying peel defect using ALT and TFL fabricated chimeric flap. Example Rep. Plast. Surg. Hand Surg. 6, 82–85. doi: 10.1080/23320885.2019.1635023
PubMed Abstruse | CrossRef Full Text | Google Scholar
Atesok, K., Doral, M. Due north., Karlsson, J., Egol, K. A., Jazrawi, 50. Yard., Coelho, P. G., et al. (2016). Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 24, 2365–2373. doi: ten.1007/s00167-014-3453-z
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bellincampi, L. D., Closkey, R. F., Prasad, R., Zawadsky, J. P., and Dunn, Yard. G. (1998). Viability of fibroblast-seeded ligament analogs after autogenous implantation. J. Orthop. Res. 16, 414–420. doi: 10.1002/jor.1100160404
PubMed Abstract | CrossRef Full Text | Google Scholar
Bi, F., Shi, Z., Liu, A., Guo, P., and Yan, S. (2015). Anterior cruciate ligament reconstruction in a rabbit model using silk-collagen scaffold and comparison with autograft. PLoS 1 ten:e0125900. doi: x.1371/journal.pone.0125900
PubMed Abstract | CrossRef Full Text | Google Scholar
Cai, J., Wang, J., Ye, K., Li, D., Ai, C., Sheng, D., et al. (2018). Dual-layer aligned-random nanofibrous scaffolds for improving slope microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int. J. Nanomed. xiii, 3481–3492. doi: 10.2147/IJN.S165633
PubMed Abstract | CrossRef Total Text | Google Scholar
Cao, Y., Liu, Y., Liu, W., Shan, Q., Buonocore, S. D., and Cui, 50. (2002). Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast. Reconstr. Surg. 110, 1280–1289. doi: 10.1097/01.PRS.0000025290.49889.4D
PubMed Abstruse | CrossRef Full Text | Google Scholar
Chen, C., Liu, F., Tang, Y. F., Qu, J., Cao, Y., Zheng, C., et al. (2019). Volume-shaped acellular fibrocartilage scaffold with cell-loading capability and chondrogenic inducibility for tissue-engineered fibrocartilage and bone-tendon healing. ACS Appl. Mater. Interfaces 11, 2891–2907. doi: ten.1021/acsami.8b20563
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, P., Cui, L., Chen, G., You, T., Li, West., Zuo, J., et al. (2019). The application of BMP-12-overexpressing mesenchymal stem cells loaded 3D-printed PLGA scaffolds in rabbit rotator cuff repair. Int. J. Biol. Macromol. 138, 79–88. doi: 10.1016/j.ijbiomac.2019.07.041
PubMed Abstract | CrossRef Full Text | Google Scholar
Chou, Y. C., Yeh, W. L., Chao, C. Fifty., Hsu, Y. H., Yu, Y. H., Chen, J. Grand., et al. (2016). Enhancement of tendon-bone healing via the combination of biodegradable collagen-loaded nanofibrous membranes and a three-dimensional printed bone-anchoring bolt. Int. J. Nanomed. 11, 4173–4186. doi: 10.2147/IJN.S108939
CrossRef Full Text | Google Scholar
Chung, E. J., Sugimoto, Chiliad. J., Koh, J. L., and Ameer, M. A. (2017). A biodegradable tri-component graft for anterior cruciate ligament reconstruction. J. Tissue Eng. Regen. Med. xi, 704–712. doi: 10.1002/term.1966
PubMed Abstract | CrossRef Full Text | Google Scholar
Crair, 1000. C., Gillespie, D. C., and Stryker, M. P. (1998). The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570. doi: 10.1126/science.279.5350.566
PubMed Abstract | CrossRef Full Text | Google Scholar
Devin, J. E., Attawia, One thousand. A., and Laurencin, C. T. (1996). Three-dimensional degradable porous polymer-ceramic matrices for use in os repair. J. Biomater. Sci. Polym. Ed. vii, 661–669. doi: x.1163/156856296X00435
PubMed Abstract | CrossRef Total Text | Google Scholar
Doty, J., Phisitkul, P., Xiao, W., Cooper, M. T., and Brigido, Southward. A. (2019). Update on acute achilles tendon injuries. Foot Talocrural joint Spec. 12, 278–280. doi: 10.1177/1938640019856804
PubMed Abstract | CrossRef Total Text | Google Scholar
Dunshee, 50. C., Sullivan, One thousand. O., and Kiick, K. Fifty. (2020). Manipulation of the dually thermoresponsive behavior of peptide-based vesicles through modification of collagen-like peptide domains. Bioeng. Transl. Med. v:e10145. doi: x.1002/btm2.10145
PubMed Abstruse | CrossRef Full Text | Google Scholar
Duty, A. O., Oest, Grand. Due east., and Guldberg, R. East. (2007). Cyclic mechanical compression increases mineralization of prison cell-seeded polymer scaffolds in vivo. J. Biomech. Eng. 129, 531–539. doi: ten.1115/one.2746375
PubMed Abstract | CrossRef Full Text | Google Scholar
Fleming, B. C., Spindler, M. P., Palmer, M. P., Magarian, E. M., and Murray, M. Chiliad. (2009). Collagen-platelet composites meliorate the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am. J. Sports Med. 37, 1554–1563. doi: 10.1177/0363546509332257
PubMed Abstract | CrossRef Total Text | Google Scholar
Fujimaki, H., Uchida, Yard., Inoue, G., Matsushita, O., Nemoto, N., Miyagi, M., et al. (2020). Polyglycolic acid-collagen tube combined with collagen-binding basic fibroblast growth factor accelerates gait recovery in a rat sciatic nerve critical-size defect model. J. Biomed. Mater. Res. B Appl. Biomater. 108, 326–332. doi: x.1002/jbm.b.34391
PubMed Abstract | CrossRef Full Text | Google Scholar
Hagg, T., Gulati, A. M., Behzadian, M. A., Vahlsing, H. Fifty., Varon, Southward., and Manthorpe, M. (1991). Nerve growth-factor promotes Cns cholinergic axonal regeneration into acellular peripheral-nerve grafts. Exp. Neurol. 112, 79–88. doi: 10.1016/0014-4886(91)90116-T
PubMed Abstract | CrossRef Total Text | Google Scholar
Han, F., Zhang, P., Chen, T., Lin, C., Wen, X., and Zhao, P. (2019). A LbL-assembled bioactive blanket modified nanofibrous membrane for rapid tendon-bone healing in ACL reconstruction. Int. J. Nanomed. 14, 9159–9172. doi: ten.2147/IJN.S214359
PubMed Abstruse | CrossRef Full Text | Google Scholar
Han, F., Zhang, P., Sun, Y., Lin, C., Zhao, P., and Chen, J. (2015). Hydroxyapatite-doped polycaprolactone nanofiber membrane improves tendon-os interface healing for anterior cruciate ligament reconstruction. Int. J. Nanomed. 10, 7333–7343. doi: 10.2147/IJN.S92099
PubMed Abstract | CrossRef Full Text | Google Scholar
Han, L., Fang, Westward. Fifty., Jin, B., Xu, Southward. C., Zheng, 10., and Hu, Y. Thousand. (2019). Enhancement of tendon-os healing later rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur. Rev. Med. Pharmacol. Sci. 23, 9075–9084. doi: 10.26355/eurrev_201910_19310
PubMed Abstract | CrossRef Full Text | Google Scholar
Harris, L. D., Kim, B. S., and Mooney, D. J. (1998). Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 42, 396-402. doi: x.1002/(SICI)1097-4636(19981205)42:3<396::AID-JBM7>3.0.CO;ii-E
PubMed Abstract | CrossRef Total Text | Google Scholar
Hayashida, K., Saijo, H., and Fujioka, Chiliad. (2018). Peroneal perforator-based peroneus longus tendon and sural neurofasciocutaneous composite flap transfer for a large soft-tissue defect of the forearm: a case report. Microsurgery 38, 85–88. doi: 10.1002/micr.30104
PubMed Abstract | CrossRef Full Text | Google Scholar
Hejbol, E. K., Sellathurai, J., Nair, P. D., and Schroder, H. D. (2017). Injectable scaffold materials differ in their prison cell instructive effects on primary human being myoblasts. J. Tissue Eng. 8:2041731417717677. doi: x.1177/2041731417717677
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, Y., Ran, J., Zheng, Z., Jin, Z., Chen, X., Yin, Z., et al. (2018). Exogenous stromal derived factor-1 releasing silk scaffold combined with intra-articular injection of progenitor cells promotes bone-ligament-bone regeneration. Acta Biomater. 71, 168–183. doi: 10.1016/j.actbio.2018.02.019
PubMed Abstruse | CrossRef Full Text | Google Scholar
Huang, Y., Pan, M., Shu, H., He, B., Zhang, F., and Lord's day, Fifty. (2020). Vascular endothelial growth factor enhances tendon-bone healing by activating Yes-associated protein for angiogenesis induction and rotator gage reconstruction in rats. J. Cell Biochem. 121, 2343–2353. doi: 10.1002/jcb.29457
PubMed Abstract | CrossRef Full Text | Google Scholar
Iwahashi, T., Shino, Thou., Nakata, Grand., Otsubo, H., Suzuki, T., Amano, H., et al. (2010). Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy 26, S13–20. doi: x.1016/j.arthro.2010.01.023
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang, J., Wan, F., Yang, J., Hao, W., Wang, Y., Yao, J., et al. (2014). Enhancement of osseointegration of polyethylene terephthalate artificial ligament by coating of silk fibroin and depositing of hydroxyapatite. Int. J. Nanomed. ix, 4569–4580. doi: 10.2147/IJN.S69137
PubMed Abstruse | CrossRef Full Text | Google Scholar
Karel, Due south., Sogorkova, J., Hermannova, 1000., Nesporova, G., Marholdova, L., Chmelickova, K., et al. (2018). Stabilization of hyaluronan-based materials by peptide conjugation and its employ as a cell-seeded scaffold in tissue applied science. Carbohydr. Polym. 201, 300–307. doi: ten.1016/j.carbpol.2018.08.082
PubMed Abstract | CrossRef Total Text | Google Scholar
Khoo, R., and Nikkhah, D. (2019). Flexor tendon retrieval of multidigit flexor zone ii injuries using push button-pull technique: video and technical considerations. Plast. Reconstr. Surg. 144, 1129e−1130e. doi: 10.1097/PRS.0000000000006285
PubMed Abstract | CrossRef Full Text | Google Scholar
Kikuchi, D., Iizuka, T., Nomura, K., Kuribayashi, Y., Tanaka, Yard., Yamashita, Due south., et al. (2018). Feasibility of autologous fibrin glue and polyglycolic acid sheets to prevent delayed haemorrhage after endoscopic submucosal dissection of gastric neoplasms in patients receiving antithrombotic therapy. Gastroenterol. Res. Pract. 2018:2174957. doi: 10.1155/2018/2174957
PubMed Abstract | CrossRef Total Text | Google Scholar
Kovacevic, D., Gulotta, L. Five., Ying, L., Ehteshami, J. R., Deng, X. H., and Rodeo, South. A. (2015). rhPDGF-BB promotes early on healing in a rat rotator cuff repair model. Clin. Orthop. Relat. Res. 473, 1644–1654. doi: 10.1007/s11999-014-4020-0
PubMed Abstract | CrossRef Total Text | Google Scholar
Kunze, 1000. Northward., Burnett, R. A., Shinsako, One thousand. K., Bush-Joseph, C. A., Cole, B. J., and Chahla, J. (2019). Trapezoidal achilles tendon allograft plug for revision quadriceps tendon repair with a big tendon defect. Arthrosc. Tech. viii, e1031–e1036. doi: 10.1016/j.eats.2019.05.015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Larn, G. D., Mcclellan, P. Eastward., Knapik, D. One thousand., Cumsky, J. 50., Webster-Forest, V., Anderson, J. Grand., et al. (2019). Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 107, 1864–1876. doi: 10.1002/jbm.b.34279
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, Grand. W., Lee, J. S., Jang, J. W., Shim, Y. B., and Lee, K. I. (2017). Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular os tunnel model. J. Tissue Eng. Regen. Med. eleven, 1435–1441. doi: 10.1002/term.2041
PubMed Abstract | CrossRef Total Text | Google Scholar
Lewandowska-Lancucka, J., Fiejdasz, S., Rodzik, L., Koziel, Yard., and Nowakowska, K. (2015). Bioactive hydrogel-nanosilica hybrid materials: a potential injectable scaffold for os tissue technology. Biomed. Mater. ten:015020. doi: ten.1088/1748-6041/10/1/015020
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, H., Chen, Y., and Chen, S. (2019). Enhancement of rotator cuff tendon-bone healing using os marrow-stimulating technique forth with hyaluronic acid. J. Orthop. Translat. 17, 96–102. doi: 10.1016/j.jot.2019.01.001
PubMed Abstract | CrossRef Total Text | Google Scholar
Li, H., Ge, Y., Zhang, P., Wu, L., and Chen, Southward. (2012). The issue of layer-past-layer chitosan-hyaluronic acid coating on graft-to-os healing of a poly(ethylene terephthalate) artificial ligament. J. Biomater. Sci. Polym. Ed. 23, 425–438. doi: 10.1163/092050610X551989
PubMed Abstruse | CrossRef Full Text | Google Scholar
Li, Ten., Cheng, R., Sun, Z., Su, W., Pan, G., Zhao, S., et al. (2017). Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 61, 204–216. doi: 10.1016/j.actbio.2017.07.044
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X., He, J., Bian, W., Li, Z., Li, D., and Snedeker, J. Yard. (2014). A novel silk-TCP-PEEK construct for anterior cruciate ligament reconstruction: an off-the shelf alternative to a bone-tendon-bone autograft. Biofabrication 6:015010. doi: 10.1088/1758-5082/vi/ane/015010
PubMed Abstruse | CrossRef Total Text | Google Scholar
Lim, W. L., Liau, L. 50., Ng, M. H., Chowdhury, S. R., and Law, J. X. (2019). Current progress in tendon and ligament tissue engineering. Tissue Eng. Regen. Med. 16, 549–571. doi: 10.1007/s13770-019-00196-west
PubMed Abstract | CrossRef Full Text | Google Scholar
Lipner, J., Shen, H., Cavinatto, L., Liu, West. Y., Havlioglu, N., Xia, Y. North., et al. (2015). In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-os repair. Tissue Eng. Part A 21, 2766–2774. doi: 10.1089/x.tea.2015.0101
PubMed Abstruse | CrossRef Full Text | Google Scholar
Liu, H. H., Yang, 50., Zhang, E. C., Zhang, R., Cai, D. D., Zhu, S. A., et al. (2017). Biomimetic tendon extracellular matrix blended slope scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 56, 129–140. doi: 10.1016/j.actbio.2017.05.027
PubMed Abstract | CrossRef Full Text | Google Scholar
Loeffler, B. J., Scannell, B. P., Peindl, R. D., Connor, P., Davis, D. E., Hoelscher, Thousand. L., et al. (2013). Prison cell-based tissue technology augments tendon-to-bone healing in a rat supraspinatus model. J. Orthop. Res. 31, 407–412. doi: 10.1002/jor.22234
PubMed Abstract | CrossRef Full Text | Google Scholar
Lu, H. B., Tang, Y. F., Liu, F., Xie, South. S., Qu, J., and Chen, C. (2019). Comparative evaluation of the book-blazon acellular bone scaffold and fibrocartilage scaffold for bone-tendon healing. J. Orthop. Res. 37, 1709–1722. doi: 10.1002/jor.24301
PubMed Abstract | CrossRef Full Text | Google Scholar
Maher, C. M., Sherrington, C., Herbert, R. D., Moseley, A. M., and Elkins, Yard. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83, 713–721. doi: 10.1093/ptj/83.8.713
PubMed Abstract | CrossRef Full Text | Google Scholar
Mikos, A. G., Sarakinos, M., Leite, S. M., Vacanti, J. P., and Langer, R. (1993). Laminated three-dimensional biodegradable foams for use in tissue technology. Biomaterials xiv, 323–330. doi: 10.1016/0142-9612(93)90049-eight
PubMed Abstract | CrossRef Full Text | Google Scholar
Mochizuki, T., Fujishiro, H., Nimura, A., Mahakkanukrauh, P., Yasuda, Thou., Muneta, T., et al. (2014). Anatomic and histologic analysis of the mid-substance and fan-similar extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral zipper. Articulatio genus Surg. Sports Traumatol. Arthrosc. 22, 336–344. doi: 10.1007/s00167-013-2404-4
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mochizuki, T., Muneta, T., Nagase, T., Shirasawa, S., Akita, Yard. I., and Sekiya, I. (2006). Cadaveric knee observation study for describing anatomic femoral tunnel placement for two-parcel inductive cruciate ligament reconstruction. Arthroscopy 22, 356–361. doi: 10.1016/j.arthro.2005.09.020
PubMed Abstract | CrossRef Total Text | Google Scholar
Mochizuki, Y., and Ochi, M. (2015). Clinical results of arthroscopic polyglycolic acid canvas patch graft for irreparable rotator gage tears. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. ii, 31–35. doi: 10.1016/j.asmart.2014.xi.002
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mohammed, D., Pardon, G., Versaevel, K., Bruyere, C., Alaimo, L., Luciano, One thousand., et al. (2020). Producing collagen micro-stripes with aligned fibers for cell migration assays. Cell Mol. Bioeng. xiii, 87–98. doi: x.1007/s12195-019-00600-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Periasamy, M., Venkatramani, H., and Shanmuganathan, R. S. (2019). Management of chronic achilles tendon injuries-review of electric current protocols and surgical options. Indian J. Plast Surg. 52, 109–116. doi: 10.1055/s-0039-1687923
PubMed Abstract | CrossRef Full Text | Google Scholar
Petruskevicius, J., Nielsen, S., Kaalund, S., Knudsen, P. R., and Overgaard, South. (2002). No effect of osteoset, a bone graft substitute, on bone healing in humans: a prospective randomized double-bullheaded study. Acta Orthop. Scand. 73, 575–578. doi: 10.1080/000164702321022875
PubMed Abstract | CrossRef Full Text | Google Scholar
Ravichandran, A., Lim, J., Chong, M. S. Yard., Wen, F., Liu, Y., Pillay, Y. T., et al. (2017). In vitro cyclic compressive loads potentiate early osteogenic events in engineered bone tissue. J. Biomed. Mater. Res. B Appl. Biomater. 105, 2366–2375. doi: x.1002/jbm.b.33772
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rodriguez-Vazquez, M., and Ramos-Zuniga, R. (2020). Chitosan-hydroxyapatite scaffold for tissue engineering in experimental lumbar laminectomy and posterolateral spinal fusion in wistar rats. Asian Spine J. 14, 139–147. doi: 10.31616/asj.2019.0091
PubMed Abstract | CrossRef Total Text | Google Scholar
Sadeghinia, A., Soltani, S., Aghazadeh, M., Khalilifard, J., and Davaran, S. (2020). Design and fabrication of clinoptilolite-nanohydroxyapatite/chitosan-gelatin composite scaffold and evaluation of its furnishings on bone tissue applied science. J. Biomed. Mater. Res. A 108, 221–233. doi: 10.1002/jbm.a.36806
PubMed Abstract | CrossRef Full Text | Google Scholar
Sasaki, N., Ishibashi, Y., Tsuda, Due east., Yamamoto, Y., Maeda, S., Mizukami, H., et al. (2012). The femoral insertion of the anterior cruciate ligament: discrepancy betwixt macroscopic and histological observations. Arthroscopy 28, 1135–1146. doi: 10.1016/j.arthro.2011.12.021
PubMed Abstract | CrossRef Full Text | Google Scholar
Serre, C. One thousand., Papillard, G., Chavassieux, P., Voegel, J. C., and Boivin, G. (1998). Influence of magnesium substitution on a collagen-apatite biomaterial on the production of a calcifying matrix by human osteoblasts. J. Biomed. Mater. Res. 42, 626–633. doi: 10.1002/(SICI)1097-4636(19981215)42:4<626::Help-JBM20>3.0.CO;2-Southward
PubMed Abstract | CrossRef Full Text | Google Scholar
Sharma, V., Kumar, A., Puri, K., Bansal, M., and Khatri, Yard. (2019). Application of platelet-rich fibrin membrane and collagen dressing as palatal cast for wound healing: a randomized clinical control trial. Indian J. Dent. Res. 30, 881–888. doi: 10.4103/ijdr.IJDR_370_17
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi, Y., Helary, C., and Coradin, T. (2019). Exploring the prison cell-protein-mineral interfaces: interplay of silica (nano)rods@collagen biocomposites with man dermal fibroblasts. Mater. Today Bio. one:100004. doi: ten.1016/j.mtbio.2019.100004
PubMed Abstract | CrossRef Total Text | Google Scholar
Szlazak, K., Vass, Five., Hasslinger, P., Jaroszewicz, J., Dejaco, A., Idaszek, J., et al. (2019). X-ray physics-based CT-to-composition conversion applied to a tissue engineering scaffold, enabling multiscale simulation of its elastic behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 95, 389–396. doi: x.1016/j.msec.2017.11.044
PubMed Abstract | CrossRef Full Text | Google Scholar
Tabuchi, Yard., Soejima, T., Kanazawa, T., Noguchi, Thousand., and Nagata, Grand. (2012). Chronological changes in the collagen-type composition at tendon-bone interface in rabbits. Bone Joint Res. one, 218–224. doi: x.1302/2046-3758.19.2000109
PubMed Abstract | CrossRef Full Text | Google Scholar
Tang, Y., Chen, C., Liu, F., Xie, S., Qu, J., Li, M., et al. (2020). Structure and ingredient-based biomimetic scaffolds combining with autologous os marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials 241:119837. doi: 10.1016/j.biomaterials.2020.119837
PubMed Abstruse | CrossRef Full Text | Google Scholar
Thian, E. S., Huang, J., Best, S. 1000., Hairdresser, Z. H., Brooks, R. A., Rushton, N., et al. (2006). The response of osteoblasts to nanocrystalline silicon-substituted hydroxyapatite thin films. Biomaterials 27, 2692–2698. doi: 10.1016/j.biomaterials.2005.12.019
PubMed Abstract | CrossRef Total Text | Google Scholar
Tonndorf, R., Aibibu, D., and Cherif, C. (2020). Corrigendum to "collagen multifilament spinning" [Mater. Sci. Eng. C. 106 (2020): 110105]. Mater. Sci. Eng. C Mater. Biol. Appl. 109:110513. doi: 10.1016/j.msec.2019.110513
CrossRef Full Text | Google Scholar
Uz, U., Gunhan, Grand., Vatansever, South., Kivanc, Thousand., and Yuceturk, A. V. (2019). Novel simple strategy for cartilage tissue engineering using stalk cells and synthetic polymer scaffold. J. Craniofac. Surg. 30, 940–943. doi: 10.1097/SCS.0000000000005374
PubMed Abstruse | CrossRef Total Text | Google Scholar
Van Der Made, A. D., Tol, J. 50., Reurink, G., Peters, R. W., and Kerkhoffs, G. M. (2019). Potential hamstring injury blind spot: we need to heighten awareness of proximal hamstring tendon avulsion injuries. Br. J. Sports Med. 53, 390–392. doi: ten.1136/bjsports-2018-100063
PubMed Abstract | CrossRef Full Text | Google Scholar
Vavken, P., Fleming, B. C., Mastrangelo, A. North., Machan, J. T., and Murray, M. M. (2012). Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy 28, 672–680. doi: ten.1016/j.arthro.2011.10.008
PubMed Abstruse | CrossRef Full Text | Google Scholar
Weiler, A., Hoffmann, R. F., Bail, H. J., Rehm, O., and Sudkamp, Northward. P. (2002). Tendon healing in a bone tunnel. Role Two: histologic assay later biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy 18, 124–135. doi: 10.1053/jars.2002.30657
PubMed Abstruse | CrossRef Total Text | Google Scholar
Wisbech Vange, Due south., Tranum-Jensen, J., and Krogsgaard, One thousand. R. (2020). Gracilis tendon harvest may lead to both incisional and not-incisional saphenous nerve injuries. Genu Surg. Sports Traumatol. Arthrosc. 28, 969–974. doi: x.1007/s00167-019-05605-0
PubMed Abstract | CrossRef Total Text | Google Scholar
Xu, Q., Sunday, Westward. X., and Zhang, Z. F. (2019). High expression of VEGFA in MSCs promotes tendon-bone healing of rotator gage tear via microRNA-205-5p. Eur. Rev. Med. Pharmacol. Sci 23, 4081–4088. doi: 10.26355/eurrev_201905_17909
PubMed Abstract | CrossRef Full Text | Google Scholar
Young, R. K., Butler, D. L., Weber, W., Caplan, A. I., Gordon, S. L., and Fink, D. J. (1998). Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. xvi, 406–413. doi: ten.1002/jor.1100160403
CrossRef Full Text | Google Scholar
Zhang, C., Wang, X. L., Zhang, E. C., Yang, L., Yuan, H. H., Tu, Due west. J., et al. (2018). An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 66, 141–156. doi: 10.1016/j.actbio.2017.09.036
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhang, P., Han, F., Chen, T., Wu, Z., and Chen, S. (2020). "Swiss scroll"-like bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-os healing after inductive cruciate ligament reconstruction. Biomater. Sci. 8, 871–883. doi: 10.1039/C9BM01703H
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, W., Pan, Westward., Zhang, M., and Wei, Y. (2011). In vivo evaluation of two types of bioactive scaffold used for tendon-bone interface healing in the reconstruction of anterior cruciate ligament. Biotechnol. Lett. 33, 837–844. doi: 10.1007/s10529-010-0490-seven
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhao, F., Hu, X., Zhang, J., Shi, W., Ren, B., Huang, H., et al. (2019). A more flattened bone tunnel has a positive result on tendon-bone healing in the early on menstruation afterward ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 27, 3543–3551. doi: 10.1007/s00167-019-05420-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhao, S., Xie, X., Pan, G., Shen, P., Zhao, J., and Cui, Due west. (2015a). Healing improvement afterward rotator cuff repair using gelatin-grafted poly(L-lactide) electrospun fibrous membranes. J. Surg. Res. 193, 33–42. doi: 10.1016/j.jss.2014.08.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhao, S., Zhao, J., Dong, S., Huangfu, X., Li, B., Yang, H., et al. (2014). Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 9, 2373–2385. doi: ten.2147/IJN.S59536
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhao, S., Zhao, X., Dong, South., Yu, J., Pan, Chiliad., Zhang, Y., et al. (2015b). A hierarchical, stretchable and stiff fibrous biotemplate engineered using stagger-electrospinning for augmentation of rotator cuff tendon-healing. J. Mater. Chem. B three, 990–1000. doi: 10.1039/C4TB01642D
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhou, Y., Xie, S., Tang, Y., Li, X., Cao, Y., Hu, J., et al. (2021). Effect of book-shaped acellular tendon scaffold with bone marrow mesenchymal stalk cells sheets on bone-tendon interface healing. J. Orthop. Trans. 26, 162–170. doi: x.1016/j.jot.2020.02.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhu, M., Tay, M. L., Callon, Thou., Tuari, D., Zhao, L., Dray, M., et al. (2019). Overlay repair with a synthetic collagen scaffold improves the quality of healing in a rat rotator cuff repair model. J. Shoulder Elbow Surg. 28, 949–958. doi: ten.1016/j.jse.2018.11.044
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhu, Q., Ma, Z. J., Li, H. Y., Wang, H. Chiliad., and He, Y. H. (2019). Enhancement of rotator cuff tendon-bone healing using combined aligned electrospun fibrous membranes and kartogenin. Rsc. Adv. 9, 15582–15592. doi: ten.1039/C8RA09849B
CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fbioe.2021.621483/full
0 Response to "Tissue Engineering for Anterior Cruciate Ligament Reconstruction a Review of Current Strategies"
إرسال تعليق